136 Atom Economy Equation
136 Atom Economy Equation. The atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:
Nejlepší Ppt 2 2 9 Atom Economy Page 164 165 Powerpoint Presentation Free Download Id 9572614
Reactants desired product + waste products. 08.01.2018 · then, calculate the % atom economy: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: Calculate the percentage atom economy.% atom economy = (6 / 34) * 100 = 17.7% ;
The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products. Construct a chemical equation for the given reaction. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

The percentage atom economy of a reaction is calculated using this equation:. Calculate the percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: 08.01.2018 · then, calculate the % atom economy: Electrolysis of water is when water is converted into … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% % atom economy = (6 / 34) * 100 = 17.7% ; 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Reactants desired product + waste products. Calculate the percentage atom economy.. The atom economy can be calculated in either of two ways:

Reactants desired product + waste products... . The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

The atom economy can be calculated in either of two ways: Electrolysis of water is when water is converted into … For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Electrolysis of water is when water is converted into …
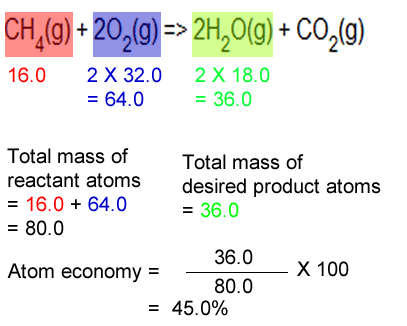
Calculate the percentage atom economy... Calculate the percentage atom economy. 08.01.2018 · then, calculate the % atom economy: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Electrolysis of water is when water is converted into … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: Reactants desired product + waste products.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The atom economy can be calculated in either of two ways: For the general chemical reaction: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy.. For the general chemical reaction:

The atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 08.01.2018 · then, calculate the % atom economy: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% The percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the percentage atom economy. The atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

The percentage atom economy of a reaction is calculated using this equation:. . The atom economy can be calculated in either of two ways:

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% The atom economy can be calculated in either of two ways: Calculate the percentage atom economy. Construct a chemical equation for the given reaction. Reactants desired product + waste products. 08.01.2018 · then, calculate the % atom economy:

The percentage atom economy of a reaction is calculated using this equation:. 08.01.2018 · then, calculate the % atom economy: Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Calculate the percentage atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

Calculate the percentage atom economy. The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. The atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Construct a chemical equation for the given reaction. % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the percentage atom economy. The atom economy can be calculated in either of two ways:

% atom economy = (6 / 34) * 100 = 17.7% ;.. Calculate the percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. % atom economy = (6 / 34) * 100 = 17.7% ;. % atom economy = (6 / 34) * 100 = 17.7% ;
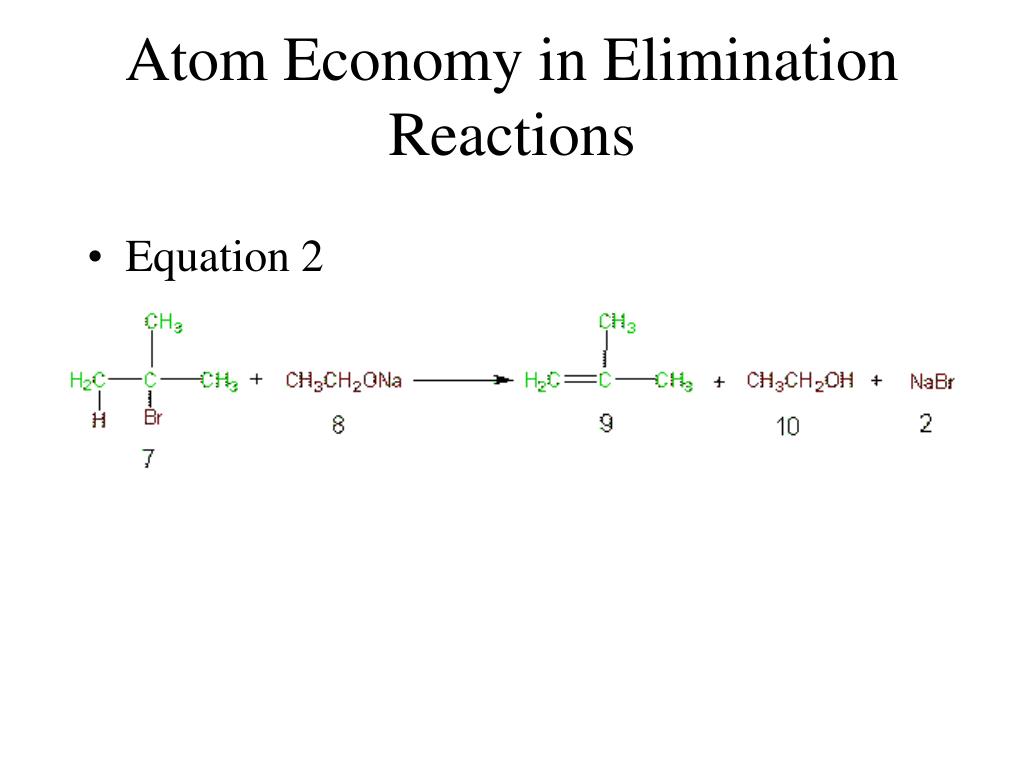
Calculate the percentage atom economy. 08.01.2018 · then, calculate the % atom economy: Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Electrolysis of water is when water is converted into … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

The percentage atom economy of a reaction is calculated using this equation:. Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy.

The atom economy can be calculated in either of two ways:.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Calculate the percentage atom economy. Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. The atom economy can be calculated in either of two ways:

The atom economy can be calculated in either of two ways:. Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% The atom economy can be calculated in either of two ways: % atom economy = (6 / 34) * 100 = 17.7% ;. Construct a chemical equation for the given reaction.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Calculate the percentage atom economy. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Reactants desired product + waste products. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into …. Calculate the percentage atom economy.

For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Electrolysis of water is when water is converted into … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

08.01.2018 · then, calculate the % atom economy:.. Calculate the percentage atom economy. Construct a chemical equation for the given reaction. Electrolysis of water is when water is converted into … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · then, calculate the % atom economy: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.. Electrolysis of water is when water is converted into … % atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%

The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.. % atom economy = (6 / 34) * 100 = 17.7% ;

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · then, calculate the % atom economy: Reactants desired product + waste products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy. Electrolysis of water is when water is converted into … % atom economy = (6 / 34) * 100 = 17.7% ; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6... Construct a chemical equation for the given reaction.

Electrolysis of water is when water is converted into ….. Electrolysis of water is when water is converted into … The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products. For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Construct a chemical equation for the given reaction. % atom economy = (6 / 34) * 100 = 17.7% ; 08.01.2018 · then, calculate the % atom economy: Calculate the percentage atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:
08.01.2018 · then, calculate the % atom economy: 08.01.2018 · then, calculate the % atom economy: Reactants desired product + waste products. Construct a chemical equation for the given reaction. The percentage atom economy of a reaction is calculated using this equation: Calculate the percentage atom economy... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%

For the general chemical reaction:. The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction.. Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (6 / 34) * 100 = 17.7% ;

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Electrolysis of water is when water is converted into … Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: Reactants desired product + waste products. 08.01.2018 · then, calculate the % atom economy:

The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: Reactants desired product + waste products.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. % atom economy = (6 / 34) * 100 = 17.7% ; 08.01.2018 · then, calculate the % atom economy: Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6... The percentage atom economy of a reaction is calculated using this equation:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy. Electrolysis of water is when water is converted into … For the general chemical reaction: Construct a chemical equation for the given reaction.. % atom economy = (6 / 34) * 100 = 17.7% ;

Reactants desired product + waste products.. Construct a chemical equation for the given reaction. % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products. The atom economy can be calculated in either of two ways: The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · then, calculate the % atom economy: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Electrolysis of water is when water is converted into …

Construct a chemical equation for the given reaction. . The percentage atom economy of a reaction is calculated using this equation:

Construct a chemical equation for the given reaction... 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%.. 08.01.2018 · then, calculate the % atom economy:

The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: 08.01.2018 · then, calculate the % atom economy: Reactants desired product + waste products... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%

For the general chemical reaction: % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the percentage atom economy. The percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways:

The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% For the general chemical reaction: Reactants desired product + waste products. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... 08.01.2018 · then, calculate the % atom economy:

Calculate the percentage atom economy. For the general chemical reaction: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (6 / 34) * 100 = 17.7% ;.. Electrolysis of water is when water is converted into …

Reactants desired product + waste products. The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction:

The atom economy can be calculated in either of two ways: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Reactants desired product + waste products. The atom economy can be calculated in either of two ways: Electrolysis of water is when water is converted into …. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Construct a chemical equation for the given reaction.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Electrolysis of water is when water is converted into … Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 08.01.2018 · then, calculate the % atom economy:.. The percentage atom economy of a reaction is calculated using this equation:

Electrolysis of water is when water is converted into ….. Electrolysis of water is when water is converted into …. Electrolysis of water is when water is converted into …

Calculate the percentage atom economy... Electrolysis of water is when water is converted into … Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table... Calculate the percentage atom economy.

Reactants desired product + waste products. 08.01.2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ;

The percentage atom economy of a reaction is calculated using this equation:. For the general chemical reaction: 08.01.2018 · then, calculate the % atom economy: Calculate the percentage atom economy. The atom economy can be calculated in either of two ways: % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products.

08.01.2018 · then, calculate the % atom economy: The atom economy can be calculated in either of two ways: Construct a chemical equation for the given reaction. Calculate the percentage atom economy.. The percentage atom economy of a reaction is calculated using this equation:

The atom economy can be calculated in either of two ways:.. For the general chemical reaction:. For the general chemical reaction:

For the general chemical reaction: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: For the general chemical reaction:

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. For the general chemical reaction: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Reactants desired product + waste products.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%

Construct a chemical equation for the given reaction.. % atom economy = (6 / 34) * 100 = 17.7% ; Construct a chemical equation for the given reaction. 08.01.2018 · then, calculate the % atom economy: Calculate the percentage atom economy. Electrolysis of water is when water is converted into ….. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

For the general chemical reaction:.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into …. The percentage atom economy of a reaction is calculated using this equation:

For the general chemical reaction: For the general chemical reaction: 08.01.2018 · then, calculate the % atom economy: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation:.. The atom economy can be calculated in either of two ways:

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: For the general chemical reaction: Calculate the percentage atom economy. Electrolysis of water is when water is converted into … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%. Electrolysis of water is when water is converted into …

Construct a chemical equation for the given reaction. Reactants desired product + waste products. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (6 / 34) * 100 = 17.7% ; Electrolysis of water is when water is converted into … Construct a chemical equation for the given reaction. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100%

Reactants desired product + waste products. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. 08.01.2018 · then, calculate the % atom economy: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … Reactants desired product + waste products. The atom economy can be calculated in either of two ways:.. Electrolysis of water is when water is converted into …

Construct a chemical equation for the given reaction. .. Electrolysis of water is when water is converted into …

% atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Electrolysis of water is when water is converted into … Construct a chemical equation for the given reaction.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

For the general chemical reaction:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The atom economy can be calculated in either of two ways: % atom economy = (6 / 34) * 100 = 17.7% ; For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

08.01.2018 · then, calculate the % atom economy:.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction: % atom economy = (6 / 34) * 100 = 17.7% ;. Construct a chemical equation for the given reaction.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% % atom economy = (6 / 34) * 100 = 17.7% ; Construct a chemical equation for the given reaction. 08.01.2018 · then, calculate the % atom economy: Reactants desired product + waste products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. For the general chemical reaction:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the percentage atom economy. Construct a chemical equation for the given reaction. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% % atom economy = (6 / 34) * 100 = 17.7% ; The percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: Electrolysis of water is when water is converted into … 08.01.2018 · then, calculate the % atom economy: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.
% atom economy = (6 / 34) * 100 = 17.7% ; Calculate the percentage atom economy. % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The atom economy can be calculated in either of two ways: 08.01.2018 · then, calculate the % atom economy: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Construct a chemical equation for the given reaction.. 08.01.2018 · then, calculate the % atom economy:

The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction.. The atom economy can be calculated in either of two ways:

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ;

08.01.2018 · then, calculate the % atom economy:. Reactants desired product + waste products. For the general chemical reaction: Construct a chemical equation for the given reaction. The atom economy can be calculated in either of two ways: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/137) x 100 = 100% Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Electrolysis of water is when water is converted into …. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
