Ideje Atom Economy Formula Chemistry Čerstvé
Ideje Atom Economy Formula Chemistry Čerstvé. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. % atom economy = (6 / 34) * 100 = 17.7% ;
Nejlepší Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry
01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Green chemists define atom economy as:The data derived can then be used to calculate the empirical formula of the original copper oxide.
Calculate the relative molecular mass of each of the products. Green chemists define atom economy as: Ch4 (g) + h2o (g) → co (g) + … Calculate the relative molecular mass of each of the products. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Construct a chemical equation for the given reaction... Atom economy = \(\frac{6}{34} \times 100\) Then, calculate the % atom economy: The reaction is as follows: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Calculate the percentage atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The data derived can then be used to calculate the empirical formula of the original copper oxide. Calculate the percentage atom economy.

Calculate the percentage atom economy... Then, calculate the % atom economy: Ch4 (g) + h2o (g) → co (g) + ….. Calculate the relative molecular mass of each of the products.
Atom economy = \(\frac{6}{34} \times 100\) 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{6}{34} \times 100\) Calculate the percentage atom economy. The reaction is as follows: Then, calculate the % atom economy: Calculate the relative molecular mass of each of the products. % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. % atom economy = (6 / 34) * 100 = 17.7% ;

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Green chemists define atom economy as:

% atom economy = (6 / 34) * 100 = 17.7% ;.. Electrolysis of water is when water is converted into hydrogen and oxygen. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. How to calculate atom economy step 1.

This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. The data derived can then be used to calculate the empirical formula of the original copper oxide. Ch4 (g) + h2o (g) → co (g) + … Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Calculate the percentage atom economy. Then, calculate the % atom economy: Finding the formula of an oxide of copper. % atom economy = (6 / 34) * 100 = 17.7% ; Construct a chemical equation for the given reaction.. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.

Calculate the relative molecular mass of each of the products. Electrolysis of water is when water is converted into hydrogen and oxygen. Ch4 (g) + h2o (g) → co (g) + … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. The reaction is as follows:

Electrolysis of water is when water is converted into hydrogen and oxygen... The reaction is as follows: How to calculate atom economy step 1. % atom economy = (6 / 34) * 100 = 17.7% ; Finding the formula of an oxide of copper.

This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Finding the formula of an oxide of copper. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Write out the balanced equation. Ch4 (g) + h2o (g) → co (g) + … The data derived can then be used to calculate the empirical formula of the original copper oxide. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table... Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Calculate the percentage atom economy. Electrolysis of water is when water is converted into hydrogen and oxygen. Calculate the relative molecular mass of each of the products. Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Then, calculate the % atom economy: Calculate the percentage atom economy.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Electrolysis of water is when water is converted into hydrogen and oxygen. Then, calculate the % atom economy: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) The reaction is as follows: Green chemists define atom economy as: Calculate the relative molecular mass of each of the products. Write out the balanced equation. % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Finding the formula of an oxide of copper. Then, calculate the % atom economy:

Calculate the relative molecular mass of each of the products. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Then, calculate the % atom economy: Calculate the percentage atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the relative molecular mass of each of the products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas.

Ch 2 =ch 2 + br 2 → ch 2 brch 2 br How to calculate atom economy step 1. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br.. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:

The data derived can then be used to calculate the empirical formula of the original copper oxide. The reaction is as follows: Green chemists define atom economy as: Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.

Finding the formula of an oxide of copper. Then, calculate the % atom economy: Electrolysis of water is when water is converted into hydrogen and oxygen. The reaction is as follows: The data derived can then be used to calculate the empirical formula of the original copper oxide. Green chemists define atom economy as: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. Construct a chemical equation for the given reaction.

Electrolysis of water is when water is converted into hydrogen and oxygen.. Then, calculate the % atom economy: The reaction is as follows: Construct a chemical equation for the given reaction. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:.. Green chemists define atom economy as:

Calculate the percentage atom economy... Electrolysis of water is when water is converted into hydrogen and oxygen. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

The reaction is as follows:.. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Then, calculate the % atom economy: Green chemists define atom economy as: Ch4 (g) + h2o (g) → co (g) + …

Then, calculate the % atom economy:. Green chemists define atom economy as: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Atom economy = \(\frac{6}{34} \times 100\) Calculate the relative molecular mass of each of the products. Finding the formula of an oxide of copper. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + …. Ch4 (g) + h2o (g) → co (g) + …

The reaction is as follows: .. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Then, calculate the % atom economy: Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The data derived can then be used to calculate the empirical formula of the original copper oxide. Calculate the relative molecular mass of each of the products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table... Green chemists define atom economy as:

% atom economy = (6 / 34) * 100 = 17.7% ; How to calculate atom economy step 1. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Construct a chemical equation for the given reaction. Calculate the relative molecular mass of each of the products. Then, calculate the % atom economy: The data derived can then be used to calculate the empirical formula of the original copper oxide. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the percentage atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Construct a chemical equation for the given reaction... Write out the balanced equation. Calculate the relative molecular mass of each of the products. % atom economy = (6 / 34) * 100 = 17.7% ;

The reaction is as follows: How to calculate atom economy step 1. Ch4 (g) + h2o (g) → co (g) + … 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Green chemists define atom economy as: Construct a chemical equation for the given reaction. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: % atom economy = (6 / 34) * 100 = 17.7% ; Atom economy = \(\frac{6}{34} \times 100\).. The reaction is as follows:

Construct a chemical equation for the given reaction. Finding the formula of an oxide of copper. % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the percentage atom economy. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 2 =ch 2 + br 2 → ch 2 brch 2 br The data derived can then be used to calculate the empirical formula of the original copper oxide. Ch4 (g) + h2o (g) → co (g) + … Construct a chemical equation for the given reaction. Calculate the relative molecular mass of each of the products. Finding the formula of an oxide of copper.

Finding the formula of an oxide of copper... Finding the formula of an oxide of copper. The data derived can then be used to calculate the empirical formula of the original copper oxide. The reaction is as follows: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch4 (g) + h2o (g) → co (g) + … Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Construct a chemical equation for the given reaction. Calculate the relative molecular mass of each of the products.

Ch4 (g) + h2o (g) → co (g) + … Write out the balanced equation. Calculate the percentage atom economy. Green chemists define atom economy as: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Finding the formula of an oxide of copper. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Green chemists define atom economy as:

Then, calculate the % atom economy: Atom economy = \(\frac{6}{34} \times 100\) Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Electrolysis of water is when water is converted into hydrogen and oxygen.

Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. How to calculate atom economy step 1. The reaction is as follows: Construct a chemical equation for the given reaction. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The data derived can then be used to calculate the empirical formula of the original copper oxide... 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

The data derived can then be used to calculate the empirical formula of the original copper oxide.. Construct a chemical equation for the given reaction. How to calculate atom economy step 1. Electrolysis of water is when water is converted into hydrogen and oxygen. The data derived can then be used to calculate the empirical formula of the original copper oxide. Green chemists define atom economy as: Calculate the relative molecular mass of each of the products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The data derived can then be used to calculate the empirical formula of the original copper oxide.

Atom economy = \(\frac{6}{34} \times 100\) Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) How to calculate atom economy step 1. Finding the formula of an oxide of copper... The reaction is as follows:

% atom economy = (6 / 34) * 100 = 17.7% ; Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Green chemists define atom economy as: Finding the formula of an oxide of copper.
Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Ch4 (g) + h2o (g) → co (g) + … Then, calculate the % atom economy: Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical... 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The reaction is as follows: Construct a chemical equation for the given reaction. Finding the formula of an oxide of copper. % atom economy = (6 / 34) * 100 = 17.7% ; Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The data derived can then be used to calculate the empirical formula of the original copper oxide. Green chemists define atom economy as: Ch4 (g) + h2o (g) → co (g) + … Finding the formula of an oxide of copper. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{6}{34} \times 100\). Green chemists define atom economy as:

Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. The data derived can then be used to calculate the empirical formula of the original copper oxide. Calculate the percentage atom economy. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Write out the balanced equation.. Then, calculate the % atom economy:
The reaction is as follows: Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Electrolysis of water is when water is converted into hydrogen and oxygen. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas.. Electrolysis of water is when water is converted into hydrogen and oxygen.

The reaction is as follows:.. . Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.

Construct a chemical equation for the given reaction. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Then, calculate the % atom economy: Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. % atom economy = (6 / 34) * 100 = 17.7% ; 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Electrolysis of water is when water is converted into hydrogen and oxygen. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Ch 2 =ch 2 + br 2 → ch 2 brch 2 br The reaction is as follows:.. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Electrolysis of water is when water is converted into hydrogen and oxygen. Then, calculate the % atom economy: Green chemists define atom economy as: Calculate the percentage atom economy. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Ch4 (g) + h2o (g) → co (g) + … How to calculate atom economy step 1. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. Write out the balanced equation.

How to calculate atom economy step 1. Atom economy = \(\frac{6}{34} \times 100\) Ch4 (g) + h2o (g) → co (g) + … Calculate the relative molecular mass of each of the products. Calculate the percentage atom economy. Calculate the percentage atom economy.

Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 2 =ch 2 + br 2 → ch 2 brch 2 br How to calculate atom economy step 1. Write out the balanced equation.

Write out the balanced equation. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. How to calculate atom economy step 1. Then, calculate the % atom economy: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. The reaction is as follows: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:
Calculate the percentage atom economy... Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Green chemists define atom economy as: The data derived can then be used to calculate the empirical formula of the original copper oxide. Construct a chemical equation for the given reaction. The reaction is as follows:.. Finding the formula of an oxide of copper.

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The data derived can then be used to calculate the empirical formula of the original copper oxide. Calculate the percentage atom economy.. Write out the balanced equation.

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Green chemists define atom economy as: How to calculate atom economy step 1. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Ch4 (g) + h2o (g) → co (g) + …. Calculate the percentage atom economy.

Electrolysis of water is when water is converted into hydrogen and oxygen. The reaction is as follows:

The data derived can then be used to calculate the empirical formula of the original copper oxide. Atom economy = \(\frac{6}{34} \times 100\) % atom economy = (6 / 34) * 100 = 17.7% ; Ch 2 =ch 2 + br 2 → ch 2 brch 2 br The data derived can then be used to calculate the empirical formula of the original copper oxide. Ch4 (g) + h2o (g) → co (g) + … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Finding the formula of an oxide of copper. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Write out the balanced equation. How to calculate atom economy step 1.. Atom economy = \(\frac{6}{34} \times 100\)
% atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Atom economy = \(\frac{6}{34} \times 100\) Electrolysis of water is when water is converted into hydrogen and oxygen. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas.

This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Then, calculate the % atom economy: Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Construct a chemical equation for the given reaction. Calculate the percentage atom economy. Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Write out the balanced equation. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
The reaction is as follows:.. How to calculate atom economy step 1. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br

Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.. Then, calculate the % atom economy: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Calculate the percentage atom economy.

Finding the formula of an oxide of copper. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Atom economy = \(\frac{6}{34} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Calculate the percentage atom economy.

Calculate the relative molecular mass of each of the products.. The data derived can then be used to calculate the empirical formula of the original copper oxide. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch4 (g) + h2o (g) → co (g) + … Green chemists define atom economy as: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Calculate the relative molecular mass of each of the products. Write out the balanced equation. Calculate the percentage atom economy. Atom economy = \(\frac{6}{34} \times 100\) Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:
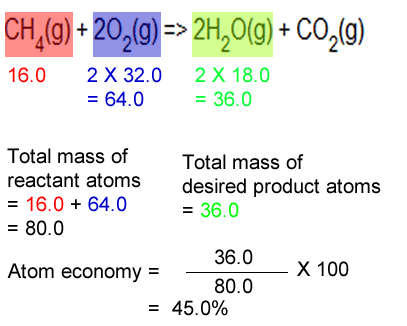
Ch4 (g) + h2o (g) → co (g) + … Construct a chemical equation for the given reaction. Atom economy = \(\frac{6}{34} \times 100\). The reaction is as follows:

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. . Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. The data derived can then be used to calculate the empirical formula of the original copper oxide. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Write out the balanced equation. Calculate the relative molecular mass of each of the products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Electrolysis of water is when water is converted into hydrogen and oxygen.

Calculate the percentage atom economy. % atom economy = (6 / 34) * 100 = 17.7% ; This resource describes the classic reduction of copper (ii) oxide to copper using methane gas... The reaction is as follows:

Ch 2 =ch 2 + br 2 → ch 2 brch 2 br 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Finding the formula of an oxide of copper. How to calculate atom economy step 1. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of.

Then, calculate the % atom economy:. Green chemists define atom economy as: % atom economy = (6 / 34) * 100 = 17.7% ; The data derived can then be used to calculate the empirical formula of the original copper oxide. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Construct a chemical equation for the given reaction. Atom economy = \(\frac{6}{34} \times 100\) Calculate the percentage atom economy. Ch4 (g) + h2o (g) → co (g) + … Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of... Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Finding the formula of an oxide of copper. The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\). This resource describes the classic reduction of copper (ii) oxide to copper using methane gas.

Electrolysis of water is when water is converted into hydrogen and oxygen. Construct a chemical equation for the given reaction. Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1.

Atom economy = \(\frac{6}{34} \times 100\).. Ch4 (g) + h2o (g) → co (g) + … How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. % atom economy = (6 / 34) * 100 = 17.7% ; 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Write out the balanced equation. Calculate the percentage atom economy. Finding the formula of an oxide of copper. Then, calculate the % atom economy: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\). Atom economy = \(\frac{6}{34} \times 100\)

% atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Ch4 (g) + h2o (g) → co (g) + … Electrolysis of water is when water is converted into hydrogen and oxygen. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Calculate the percentage atom economy. Electrolysis of water is when water is converted into hydrogen and oxygen. Calculate the percentage atom economy.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Finding the formula of an oxide of copper. % atom economy = (6 / 34) * 100 = 17.7% ;

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Construct a chemical equation for the given reaction. Ch4 (g) + h2o (g) → co (g) + … Finding the formula of an oxide of copper. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Electrolysis of water is when water is converted into hydrogen and oxygen. The data derived can then be used to calculate the empirical formula of the original copper oxide. Atom economy = \(\frac{6}{34} \times 100\) Write out the balanced equation. The reaction is as follows: % atom economy = (6 / 34) * 100 = 17.7% ;

Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Finding the formula of an oxide of copper. Calculate the relative molecular mass of each of the products. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. The data derived can then be used to calculate the empirical formula of the original copper oxide. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The reaction is as follows: Atom economy = \(\frac{6}{34} \times 100\) Write out the balanced equation. Calculate the percentage atom economy. Calculate the percentage atom economy.

Write out the balanced equation. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Green chemists define atom economy as: How to calculate atom economy step 1. Construct a chemical equation for the given reaction. Write out the balanced equation. Calculate the relative molecular mass of each of the products. The reaction is as follows: Ch4 (g) + h2o (g) → co (g) + … Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.. % atom economy = (6 / 34) * 100 = 17.7% ;

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Finding the formula of an oxide of copper. Calculate the relative molecular mass of each of the products. The reaction is as follows: Then, calculate the % atom economy: This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.. Calculate the percentage atom economy.

Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Finding the formula of an oxide of copper. The data derived can then be used to calculate the empirical formula of the original copper oxide. The reaction is as follows: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Calculate the relative molecular mass of each of the products. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Construct a chemical equation for the given reaction. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100.

Then, calculate the % atom economy:. Green chemists define atom economy as:.. Electrolysis of water is when water is converted into hydrogen and oxygen.

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Construct a chemical equation for the given reaction.

Green chemists define atom economy as:. Green chemists define atom economy as: 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Then, calculate the % atom economy: Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: The data derived can then be used to calculate the empirical formula of the original copper oxide. Write out the balanced equation. Finding the formula of an oxide of copper. Calculate the percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Write out the balanced equation.

Ch 2 =ch 2 + br 2 → ch 2 brch 2 br 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. How to calculate atom economy step 1. Write out the balanced equation. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) The reaction is as follows: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The data derived can then be used to calculate the empirical formula of the original copper oxide.. Write out the balanced equation.

The data derived can then be used to calculate the empirical formula of the original copper oxide. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. % atom economy = (6 / 34) * 100 = 17.7% ; Green chemists define atom economy as: Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Construct a chemical equation for the given reaction. Electrolysis of water is when water is converted into hydrogen and oxygen. Calculate the percentage atom economy. The reaction is as follows: How to calculate atom economy step 1.. Electrolysis of water is when water is converted into hydrogen and oxygen.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Construct a chemical equation for the given reaction. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br The reaction is as follows: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical.. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br

Calculate the percentage atom economy. Construct a chemical equation for the given reaction. Green chemists define atom economy as: Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. Atom economy = \(\frac{6}{34} \times 100\) Calculate the percentage atom economy. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Ch 2 =ch 2 + br 2 → ch 2 brch 2 br The data derived can then be used to calculate the empirical formula of the original copper oxide. % atom economy = (6 / 34) * 100 = 17.7% ; Atom economy = \(\frac{6}{34} \times 100\)

Write out the balanced equation. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Calculate the relative molecular mass of each of the products.

Write out the balanced equation... Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Green chemists define atom economy as: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

How to calculate atom economy step 1.. % atom economy = (6 / 34) * 100 = 17.7% ; Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:

Finding the formula of an oxide of copper. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The data derived can then be used to calculate the empirical formula of the original copper oxide. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100. Ch4 (g) + h2o (g) → co (g) + … Then, calculate the % atom economy: The reaction is as follows: This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Calculate the percentage atom economy. Finding the formula of an oxide of copper. Atom economy = \(\frac{6}{34} \times 100\) Calculate the percentage atom economy.

Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Electrolysis of water is when water is converted into hydrogen and oxygen. The reaction is as follows: Write out the balanced equation. Calculate the relative molecular mass of each of the products. Atom economy = \(\frac{total~m_{r}~of~the~desired~product}{total~m_{r}~of~all~reactants} \times 100\) atom economy = \(\frac{2 \times 46}{180} \times 100\) Green chemists define atom economy as: Atom economy = \(\frac{6}{34} \times 100\) Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine:. Finding the formula of an oxide of copper.

Ch 2 =ch 2 + br 2 → ch 2 brch 2 br Calculate the relative molecular mass of each of the products. Atom economy = \(\frac{6}{34} \times 100\) Ch4 (g) + h2o (g) → co (g) + … Construct a chemical equation for the given reaction. Calculate the percentage atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Green chemists define atom economy as:.. The data derived can then be used to calculate the empirical formula of the original copper oxide.

Green chemists define atom economy as: Green chemists define atom economy as: Whilst often carried out as a demonstration, this resource describes carrying out the investigation as a class practical. % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the relative molecular mass of each of the products. Ch4 (g) + h2o (g) → co (g) + … This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br. Ch 2 =ch 2 + br 2 → ch 2 brch 2 br

23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. . 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

% atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (6 / 34) * 100 = 17.7% ; Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 23.09.2012 · the atom economy of a chemical reaction is calculated using the formula atom economy = mass of useful product / mass of products x 100 this effectively gives the percentage of the mass of. Whenever there is only one product, the atom economy will always be 100% for example, in the reaction between ethene and bromine: Then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ;

How to calculate atom economy step 1. This resource describes the classic reduction of copper (ii) oxide to copper using methane gas. Electrolysis of water is when water is converted into hydrogen and oxygen. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: % atom economy = (6 / 34) * 100 = 17.7% ;
