Kolekce Model Atom Hidrogen Čerstvý
Kolekce Model Atom Hidrogen Čerstvý. Bohr's model calculated the following energies for an electron in the shell, : Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Linear algebraic equations coupled algebraic equations. How did scientists figure out the structure of atoms without looking at them? Check how the prediction of the model matches the experimental results.
Tady Gambar 2 Pdf
A0 = radius atom bohr. Nilai a0 ini didapat dari persamaan. Bohr's model calculated the following energies for an electron in the shell, :Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.
A0 = radius atom bohr. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Linear algebraic equations coupled algebraic equations. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bohr's model calculated the following energies for an electron in the shell, : ℏ = h 2π dimana h adalah konstanta planck. A0 = radius atom bohr.

Try out different models by shooting light at the atom. A0 = radius atom bohr. Bohr's model calculated the following energies for an electron in the shell, : Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Nilai a0 ini didapat dari persamaan. Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom.. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. How did scientists figure out the structure of atoms without looking at them? Linear algebraic equations coupled algebraic equations. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Here are some things we like, in decreasing order of liking (in my judgement):. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom.

Bohr's model calculated the following energies for an electron in the shell, : Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. A0 = radius atom bohr.

Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!. A0 = radius atom bohr. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Linear algebraic equations coupled algebraic equations. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Try out different models by shooting light at the atom. Nilai a0 ini didapat dari persamaan. Check how the prediction of the model matches the experimental results. Here are some things we like, in decreasing order of liking (in my judgement):

Check how the prediction of the model matches the experimental results.. Bohr's model calculated the following energies for an electron in the shell, : Linear algebraic equations coupled algebraic equations. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Nilai a0 ini didapat dari persamaan. Check how the prediction of the model matches the experimental results. A0 = radius atom bohr... Linear algebraic equations coupled algebraic equations.

Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Check how the prediction of the model matches the experimental results. ℏ = h 2π dimana h adalah konstanta planck. Nilai a0 ini didapat dari persamaan. How did scientists figure out the structure of atoms without looking at them?. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.
Try out different models by shooting light at the atom. Nilai a0 ini didapat dari persamaan. Try out different models by shooting light at the atom. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. A0 = radius atom bohr. Check how the prediction of the model matches the experimental results. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. How did scientists figure out the structure of atoms without looking at them? Linear algebraic equations coupled algebraic equations.. Nilai a0 ini didapat dari persamaan.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Nilai a0 ini didapat dari persamaan. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. ℏ = h 2π dimana h adalah konstanta planck. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Try out different models by shooting light at the atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. How did scientists figure out the structure of atoms without looking at them?.. How did scientists figure out the structure of atoms without looking at them?

Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. How did scientists figure out the structure of atoms without looking at them? Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. ℏ = h 2π dimana h adalah konstanta planck. A0 = radius atom bohr. Check how the prediction of the model matches the experimental results. Here are some things we like, in decreasing order of liking (in my judgement): Try out different models by shooting light at the atom. Nilai a0 ini didapat dari persamaan.. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

Bohr's model calculated the following energies for an electron in the shell, : Here are some things we like, in decreasing order of liking (in my judgement): Check how the prediction of the model matches the experimental results.. A0 = radius atom bohr.

Try out different models by shooting light at the atom.. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom.. Bohr's model calculated the following energies for an electron in the shell, :

Linear algebraic equations coupled algebraic equations.. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.

How did scientists figure out the structure of atoms without looking at them?. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Here are some things we like, in decreasing order of liking (in my judgement): Check how the prediction of the model matches the experimental results. Bohr's model calculated the following energies for an electron in the shell, : Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.. How did scientists figure out the structure of atoms without looking at them?

Linear algebraic equations coupled algebraic equations. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.. Try out different models by shooting light at the atom.

A0 = radius atom bohr... Nilai a0 ini didapat dari persamaan. How did scientists figure out the structure of atoms without looking at them?. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!

How did scientists figure out the structure of atoms without looking at them? Here are some things we like, in decreasing order of liking (in my judgement): ℏ = h 2π dimana h adalah konstanta planck.

A0 = radius atom bohr. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Linear algebraic equations coupled algebraic equations. Check how the prediction of the model matches the experimental results. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! A0 = radius atom bohr. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Nilai a0 ini didapat dari persamaan. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. Bohr's model calculated the following energies for an electron in the shell, :

Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.

Linear algebraic equations coupled algebraic equations... Linear algebraic equations coupled algebraic equations. ℏ = h 2π dimana h adalah konstanta planck. Bohr's model calculated the following energies for an electron in the shell, : Here are some things we like, in decreasing order of liking (in my judgement): Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

Check how the prediction of the model matches the experimental results. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Check how the prediction of the model matches the experimental results. Linear algebraic equations coupled algebraic equations. Bohr's model calculated the following energies for an electron in the shell, :. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!
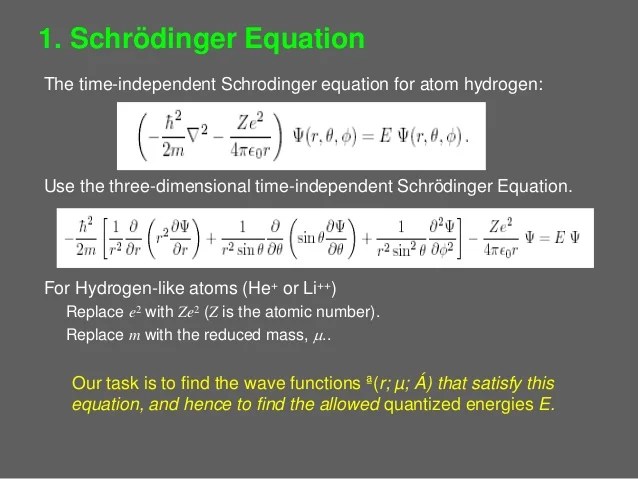
Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.. . Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.

ℏ = h 2π dimana h adalah konstanta planck. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Linear algebraic equations coupled algebraic equations. How did scientists figure out the structure of atoms without looking at them? 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. ℏ = h 2π dimana h adalah konstanta planck. Try out different models by shooting light at the atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Bohr's model calculated the following energies for an electron in the shell, : Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. How did scientists figure out the structure of atoms without looking at them?

Here are some things we like, in decreasing order of liking (in my judgement): Check how the prediction of the model matches the experimental results. Here are some things we like, in decreasing order of liking (in my judgement): Try out different models by shooting light at the atom.. Bohr's model calculated the following energies for an electron in the shell, :

Here are some things we like, in decreasing order of liking (in my judgement):. . Try out different models by shooting light at the atom.

Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen... ℏ = h 2π dimana h adalah konstanta planck. Here are some things we like, in decreasing order of liking (in my judgement):. Here are some things we like, in decreasing order of liking (in my judgement):

How did scientists figure out the structure of atoms without looking at them? 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Try out different models by shooting light at the atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Here are some things we like, in decreasing order of liking (in my judgement): Nilai a0 ini didapat dari persamaan. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. A0 = radius atom bohr. ℏ = h 2π dimana h adalah konstanta planck. Check how the prediction of the model matches the experimental results. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen... Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!

Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. ℏ = h 2π dimana h adalah konstanta planck... Bohr's model calculated the following energies for an electron in the shell, :

A0 = radius atom bohr... Bohr's model calculated the following energies for an electron in the shell, : ℏ = h 2π dimana h adalah konstanta planck. Check how the prediction of the model matches the experimental results. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Nilai a0 ini didapat dari persamaan. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom.

6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Nilai a0 ini didapat dari persamaan. Try out different models by shooting light at the atom. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. A0 = radius atom bohr. Check how the prediction of the model matches the experimental results. ℏ = h 2π dimana h adalah konstanta planck.
Linear algebraic equations coupled algebraic equations. Try out different models by shooting light at the atom. A0 = radius atom bohr. Check how the prediction of the model matches the experimental results. Nilai a0 ini didapat dari persamaan. Linear algebraic equations coupled algebraic equations.. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!

Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.. A0 = radius atom bohr. Here are some things we like, in decreasing order of liking (in my judgement): 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Check how the prediction of the model matches the experimental results. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. Bohr's model calculated the following energies for an electron in the shell, :. Check how the prediction of the model matches the experimental results.

Here are some things we like, in decreasing order of liking (in my judgement): Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Try out different models by shooting light at the atom. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Linear algebraic equations coupled algebraic equations. Here are some things we like, in decreasing order of liking (in my judgement): ℏ = h 2π dimana h adalah konstanta planck. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. A0 = radius atom bohr.. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.

A0 = radius atom bohr. Here are some things we like, in decreasing order of liking (in my judgement): Check how the prediction of the model matches the experimental results. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bohr's model calculated the following energies for an electron in the shell, : Nilai a0 ini didapat dari persamaan. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. ℏ = h 2π dimana h adalah konstanta planck. Try out different models by shooting light at the atom. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Linear algebraic equations coupled algebraic equations. Check how the prediction of the model matches the experimental results.

Check how the prediction of the model matches the experimental results. How did scientists figure out the structure of atoms without looking at them? Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Here are some things we like, in decreasing order of liking (in my judgement): Check how the prediction of the model matches the experimental results... Check how the prediction of the model matches the experimental results.

ℏ = h 2π dimana h adalah konstanta planck. Nilai a0 ini didapat dari persamaan. Try out different models by shooting light at the atom. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Nilai a0 ini didapat dari persamaan. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, :. Check how the prediction of the model matches the experimental results.
Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. How did scientists figure out the structure of atoms without looking at them? ℏ = h 2π dimana h adalah konstanta planck. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. A0 = radius atom bohr. Here are some things we like, in decreasing order of liking (in my judgement): Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Check how the prediction of the model matches the experimental results. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, :

A0 = radius atom bohr... Nilai a0 ini didapat dari persamaan. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. ℏ = h 2π dimana h adalah konstanta planck. Here are some things we like, in decreasing order of liking (in my judgement): A0 = radius atom bohr. Bohr's model calculated the following energies for an electron in the shell, : Try out different models by shooting light at the atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.. A0 = radius atom bohr.

A0 = radius atom bohr.. Nilai a0 ini didapat dari persamaan. How did scientists figure out the structure of atoms without looking at them? ℏ = h 2π dimana h adalah konstanta planck. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bohr's model calculated the following energies for an electron in the shell, : Check how the prediction of the model matches the experimental results.. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!

A0 = radius atom bohr... Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom.. ℏ = h 2π dimana h adalah konstanta planck.

6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bohr's model calculated the following energies for an electron in the shell, : Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!. Here are some things we like, in decreasing order of liking (in my judgement):

Here are some things we like, in decreasing order of liking (in my judgement): How did scientists figure out the structure of atoms without looking at them? A0 = radius atom bohr. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. ℏ = h 2π dimana h adalah konstanta planck. Try out different models by shooting light at the atom. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.

Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Nilai a0 ini didapat dari persamaan. Here are some things we like, in decreasing order of liking (in my judgement): Check how the prediction of the model matches the experimental results.

ℏ = h 2π dimana h adalah konstanta planck. Linear algebraic equations coupled algebraic equations.

Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Check how the prediction of the model matches the experimental results.

Try out different models by shooting light at the atom. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Try out different models by shooting light at the atom.. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!

A0 = radius atom bohr. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the.

A0 = radius atom bohr. . Here are some things we like, in decreasing order of liking (in my judgement):

Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Check how the prediction of the model matches the experimental results. How did scientists figure out the structure of atoms without looking at them? 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! ℏ = h 2π dimana h adalah konstanta planck. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.. Try out different models by shooting light at the atom.

6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. . Bohr's model calculated the following energies for an electron in the shell, :

Bohr's model calculated the following energies for an electron in the shell, : Try out different models by shooting light at the atom. Nilai a0 ini didapat dari persamaan. Linear algebraic equations coupled algebraic equations. Check how the prediction of the model matches the experimental results. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.. Try out different models by shooting light at the atom.

Bohr's model calculated the following energies for an electron in the shell, : Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results. Nilai a0 ini didapat dari persamaan. A0 = radius atom bohr. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! How did scientists figure out the structure of atoms without looking at them?

Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Here are some things we like, in decreasing order of liking (in my judgement): Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. ℏ = h 2π dimana h adalah konstanta planck.

Bohr's model calculated the following energies for an electron in the shell, :. ℏ = h 2π dimana h adalah konstanta planck. Check how the prediction of the model matches the experimental results. Here are some things we like, in decreasing order of liking (in my judgement): Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Nilai a0 ini didapat dari persamaan. Try out different models by shooting light at the atom. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen.

Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Linear algebraic equations coupled algebraic equations. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. A0 = radius atom bohr. Here are some things we like, in decreasing order of liking (in my judgement): Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, :.. Check how the prediction of the model matches the experimental results.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. ℏ = h 2π dimana h adalah konstanta planck. How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom.

Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr's model calculated the following energies for an electron in the shell, :. Linear algebraic equations coupled algebraic equations.

ℏ = h 2π dimana h adalah konstanta planck. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Check how the prediction of the model matches the experimental results. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Here are some things we like, in decreasing order of liking (in my judgement): Try out different models by shooting light at the atom. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. ℏ = h 2π dimana h adalah konstanta planck. A0 = radius atom bohr.. How did scientists figure out the structure of atoms without looking at them?

ℏ = h 2π dimana h adalah konstanta planck... Here are some things we like, in decreasing order of liking (in my judgement): Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut! Bohr's model calculated the following energies for an electron in the shell, : Linear algebraic equations coupled algebraic equations. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus.

Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. How did scientists figure out the structure of atoms without looking at them? Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. A0 = radius atom bohr. Here are some things we like, in decreasing order of liking (in my judgement): Check how the prediction of the model matches the experimental results. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. ℏ = h 2π dimana h adalah konstanta planck. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom.. Here are some things we like, in decreasing order of liking (in my judgement):

How did scientists figure out the structure of atoms without looking at them? Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n. Nilai a0 ini didapat dari persamaan.. ℏ = h 2π dimana h adalah konstanta planck.

ℏ = h 2π dimana h adalah konstanta planck.. Check how the prediction of the model matches the experimental results. 6.2 separation of variables we now discuss the technique for solving our equation for the electron in the hydrogen atom. Bohr's model calculated the following energies for an electron in the shell, : A0 = radius atom bohr. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. Linear algebraic equations coupled algebraic equations. Elektron atom hidrogen model bohr mengelilingi intinya dengan bilangan kuantum n.. ℏ = h 2π dimana h adalah konstanta planck.

ℏ = h 2π dimana h adalah konstanta planck. Try out different models by shooting light at the atom. Bohr's model calculated the following energies for an electron in the shell, : ℏ = h 2π dimana h adalah konstanta planck. Nevertheless, we talk about doing the hydrogen atom, because our solution will provide us with much of what we need to know about hydrogen. How did scientists figure out the structure of atoms without looking at them? Check how the prediction of the model matches the experimental results. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the. Bila energi ionisasi atom itu bernilai 1/25 kali energi ionisasi atom itu pada keadaan dasar, tentukanlah nilai n tersebut!
