Kolekce 38 Jj Thomson Structure Of Atom Vynikající
Kolekce 38 Jj Thomson Structure Of Atom Vynikající. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Common questions about thomson, rutherford, and atomic structure So, he proposed a model on the basis of known properties available at. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph.
Nejlepší J J Thomson Model Of An Atom Class 9 Structure Of An Atom
The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson model of an atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson was the first one to propose a model for the structure of an atom. Smallest fundamental particle is atom and atom can not be further subdivided.It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.
1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson's notion of the electron came from his work with a nineteenth century scientific … Smallest fundamental particle is atom and atom can not be further subdivided. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. So, he proposed a model on the basis of known properties available at.

The description of thomson's atomic model is one of the many scientific models of the atom. He is one of the most influential people in the atomic study. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. However, at that time the atomic nucleus was yet to be discovered. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. So, he proposed a model on the basis of known properties available at. The first scientific theory of atomic structure was proposed by john dalton in the 1800s.. Thomson model of an atom.

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson's notion of the electron came from his work with a nineteenth century scientific … Thomson was the first one to propose a model for the structure of an atom. What did thomson discover in the atom? Thomson model of an atom. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph.. The first scientific theory of atomic structure was proposed by john dalton in the 1800s.

Smallest fundamental particle is atom and atom can not be further subdivided. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson was the first one to propose a model for the structure of an atom. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. He is one of the most influential people in the atomic study. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Common questions about thomson, rutherford, and atomic structure However, at that time the atomic nucleus was yet to be discovered. Thomson's notion of the electron came from his work with a nineteenth century scientific … Thomson model of an atom.. What did thomson discover in the atom?

Smallest fundamental particle is atom and atom can not be further subdivided. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Smallest fundamental particle is atom and atom can not be further subdivided... Smallest fundamental particle is atom and atom can not be further subdivided.

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Thomson dramatically changed the modern view of the atom with his discovery of the electron.

Thomson model of an atom.. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Smallest fundamental particle is atom and atom can not be further subdivided. He is one of the most influential people in the atomic study.

However, at that time the atomic nucleus was yet to be discovered. Smallest fundamental particle is atom and atom can not be further subdivided. The description of thomson's atomic model is one of the many scientific models of the atom. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Common questions about thomson, rutherford, and atomic structure Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson was the first one to propose a model for the structure of an atom. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. However, at that time the atomic nucleus was yet to be discovered. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. This article will cover atomic theory by jj thomson, his early life and other atomic theories after.

He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.. Thomson's notion of the electron came from his work with a nineteenth century scientific …

This article will cover atomic theory by jj thomson, his early life and other atomic theories after. This article will cover atomic theory by jj thomson, his early life and other atomic theories after.

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate.. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson model of an atom. The description of thomson's atomic model is one of the many scientific models of the atom.. Smallest fundamental particle is atom and atom can not be further subdivided.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons... Thomson model of an atom. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. Smallest fundamental particle is atom and atom can not be further subdivided. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is... Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate.

Common questions about thomson, rutherford, and atomic structure 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. Thomson dramatically changed the modern view of the atom with his discovery of the electron. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson model of an atom. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. However, at that time the atomic nucleus was yet to be discovered. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. He is one of the most influential people in the atomic study.. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. So, he proposed a model on the basis of known properties available at. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. This was an amazing discovery, and the stage was set for the modern era of atomic studies. What did thomson discover in the atom? He is one of the most influential people in the atomic study.. So, he proposed a model on the basis of known properties available at.

Common questions about thomson, rutherford, and atomic structure. He is one of the most influential people in the atomic study. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson model of an atom. However, at that time the atomic nucleus was yet to be discovered. This was an amazing discovery, and the stage was set for the modern era of atomic studies. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. The description of thomson's atomic model is one of the many scientific models of the atom.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. The description of thomson's atomic model is one of the many scientific models of the atom. What did thomson discover in the atom? Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson model of an atom.
-teachoo.png)
So, he proposed a model on the basis of known properties available at. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson's notion of the electron came from his work with a nineteenth century scientific … What did thomson discover in the atom? This was an amazing discovery, and the stage was set for the modern era of atomic studies. The description of thomson's atomic model is one of the many scientific models of the atom. So, he proposed a model on the basis of known properties available at.

What did thomson discover in the atom? He is one of the most influential people in the atomic study. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson was the first one to propose a model for the structure of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This article will cover atomic theory by jj thomson, his early life and other atomic theories after... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

Thomson model of an atom. The description of thomson's atomic model is one of the many scientific models of the atom. He is one of the most influential people in the atomic study. Common questions about thomson, rutherford, and atomic structure

Thomson model of an atom. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson was the first one to propose a model for the structure of an atom. However, at that time the atomic nucleus was yet to be discovered. He is one of the most influential people in the atomic study. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. This was an amazing discovery, and the stage was set for the modern era of atomic studies. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate.. This article will cover atomic theory by jj thomson, his early life and other atomic theories after.

He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. Smallest fundamental particle is atom and atom can not be further subdivided.
However, at that time the atomic nucleus was yet to be discovered. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. He is one of the most influential people in the atomic study. Common questions about thomson, rutherford, and atomic structure The description of thomson's atomic model is one of the many scientific models of the atom. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. However, at that time the atomic nucleus was yet to be discovered. What did thomson discover in the atom?. Thomson was the first one to propose a model for the structure of an atom.
It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Smallest fundamental particle is atom and atom can not be further subdivided. Thomson model of an atom. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. This was an amazing discovery, and the stage was set for the modern era of atomic studies. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson's notion of the electron came from his work with a nineteenth century scientific … Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson was the first one to propose a model for the structure of an atom.. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.
Thomson model of an atom. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Common questions about thomson, rutherford, and atomic structure In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. The description of thomson's atomic model is one of the many scientific models of the atom. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. He is one of the most influential people in the atomic study.. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.
So, he proposed a model on the basis of known properties available at.. So, he proposed a model on the basis of known properties available at. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. What did thomson discover in the atom? Common questions about thomson, rutherford, and atomic structure In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson was the first one to propose a model for the structure of an atom. Thomson model of an atom. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom... Thomson dramatically changed the modern view of the atom with his discovery of the electron.
So, he proposed a model on the basis of known properties available at.. So, he proposed a model on the basis of known properties available at. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Thomson was the first one to propose a model for the structure of an atom. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific … Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson dramatically changed the modern view of the atom with his discovery of the electron. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Common questions about thomson, rutherford, and atomic structure.. Common questions about thomson, rutherford, and atomic structure

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. What did thomson discover in the atom? He is one of the most influential people in the atomic study. He is one of the most influential people in the atomic study.

Thomson model of an atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure.. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. Smallest fundamental particle is atom and atom can not be further subdivided. So, he proposed a model on the basis of known properties available at... So, he proposed a model on the basis of known properties available at.

Smallest fundamental particle is atom and atom can not be further subdivided. What did thomson discover in the atom? The first scientific theory of atomic structure was proposed by john dalton in the 1800s. This article will cover atomic theory by jj thomson, his early life and other atomic theories after.. The description of thomson's atomic model is one of the many scientific models of the atom.

Smallest fundamental particle is atom and atom can not be further subdivided. What did thomson discover in the atom? 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. Thomson's notion of the electron came from his work with a nineteenth century scientific …

The first scientific theory of atomic structure was proposed by john dalton in the 1800s. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. He is one of the most influential people in the atomic study. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. However, at that time the atomic nucleus was yet to be discovered... This was an amazing discovery, and the stage was set for the modern era of atomic studies.

The first scientific theory of atomic structure was proposed by john dalton in the 1800s. So, he proposed a model on the basis of known properties available at. Thomson's notion of the electron came from his work with a nineteenth century scientific ….. Thomson dramatically changed the modern view of the atom with his discovery of the electron.
.PNG)
Thomson was the first one to propose a model for the structure of an atom.. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. This was an amazing discovery, and the stage was set for the modern era of atomic studies. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific … Thomson was the first one to propose a model for the structure of an atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.
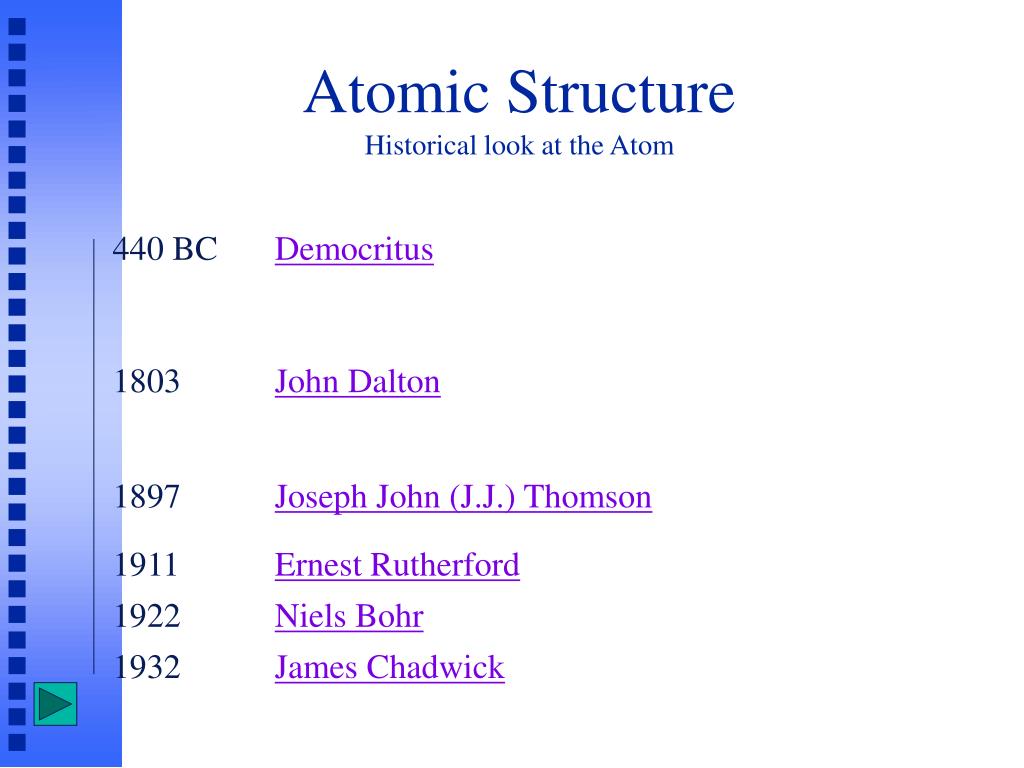
However, at that time the atomic nucleus was yet to be discovered.. Common questions about thomson, rutherford, and atomic structure It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson model of an atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. What did thomson discover in the atom? However, at that time the atomic nucleus was yet to be discovered. Smallest fundamental particle is atom and atom can not be further subdivided. What did thomson discover in the atom?

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson was the first one to propose a model for the structure of an atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This was an amazing discovery, and the stage was set for the modern era of atomic studies.

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. . The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Common questions about thomson, rutherford, and atomic structure There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure.. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

The first scientific theory of atomic structure was proposed by john dalton in the 1800s. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure.. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate.

What did thomson discover in the atom? What did thomson discover in the atom? Thomson model of an atom. Smallest fundamental particle is atom and atom can not be further subdivided. However, at that time the atomic nucleus was yet to be discovered. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson's notion of the electron came from his work with a nineteenth century scientific … It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.
Smallest fundamental particle is atom and atom can not be further subdivided.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate.. So, he proposed a model on the basis of known properties available at.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. . It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.
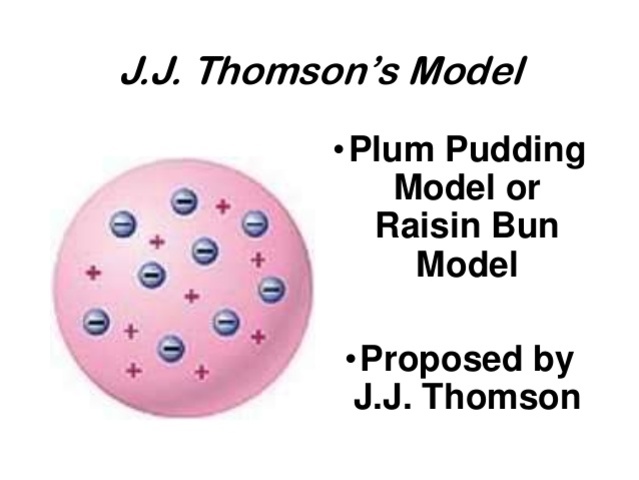
Common questions about thomson, rutherford, and atomic structure. This was an amazing discovery, and the stage was set for the modern era of atomic studies. Thomson model of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.. What did thomson discover in the atom?

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize... In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. The description of thomson's atomic model is one of the many scientific models of the atom. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. This was an amazing discovery, and the stage was set for the modern era of atomic studies. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. However, at that time the atomic nucleus was yet to be discovered. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure... The description of thomson's atomic model is one of the many scientific models of the atom.
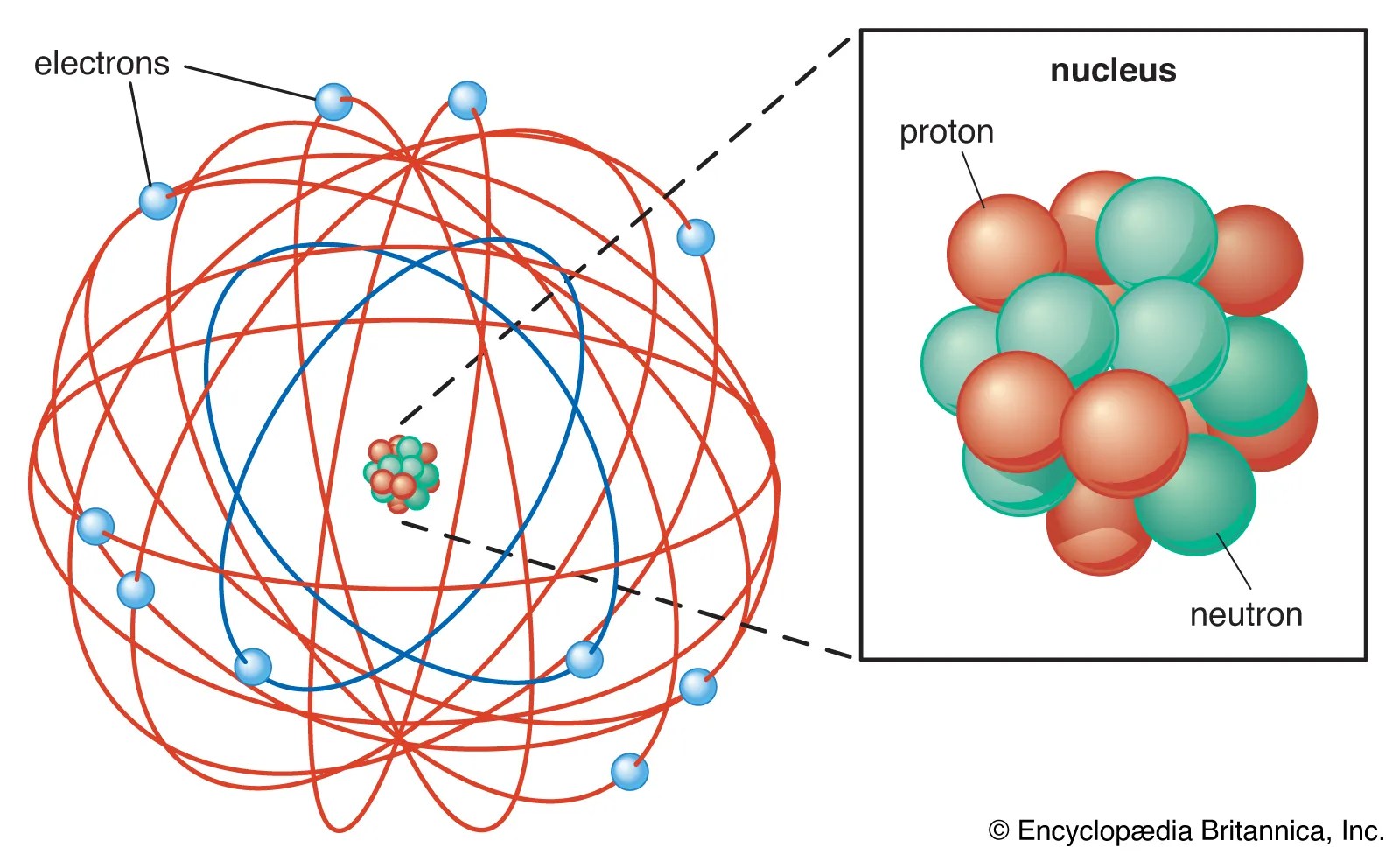
Common questions about thomson, rutherford, and atomic structure. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. However, at that time the atomic nucleus was yet to be discovered. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Common questions about thomson, rutherford, and atomic structure He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. The description of thomson's atomic model is one of the many scientific models of the atom.

The description of thomson's atomic model is one of the many scientific models of the atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson's notion of the electron came from his work with a nineteenth century scientific … However, at that time the atomic nucleus was yet to be discovered. This was an amazing discovery, and the stage was set for the modern era of atomic studies. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure.

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate.. .. This article will cover atomic theory by jj thomson, his early life and other atomic theories after.

This was an amazing discovery, and the stage was set for the modern era of atomic studies. Thomson was the first one to propose a model for the structure of an atom. However, at that time the atomic nucleus was yet to be discovered. What did thomson discover in the atom? The description of thomson's atomic model is one of the many scientific models of the atom.. Smallest fundamental particle is atom and atom can not be further subdivided.
.PNG)
This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph.. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson model of an atom. Thomson's notion of the electron came from his work with a nineteenth century scientific … He is one of the most influential people in the atomic study. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. The description of thomson's atomic model is one of the many scientific models of the atom. This article will cover atomic theory by jj thomson, his early life and other atomic theories after.

Smallest fundamental particle is atom and atom can not be further subdivided... Thomson dramatically changed the modern view of the atom with his discovery of the electron. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. So, he proposed a model on the basis of known properties available at. What did thomson discover in the atom? He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. This was an amazing discovery, and the stage was set for the modern era of atomic studies. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. Thomson model of an atom. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.

Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Common questions about thomson, rutherford, and atomic structure So, he proposed a model on the basis of known properties available at. Thomson's notion of the electron came from his work with a nineteenth century scientific … In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made.. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure.

Smallest fundamental particle is atom and atom can not be further subdivided... Smallest fundamental particle is atom and atom can not be further subdivided. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. This was an amazing discovery, and the stage was set for the modern era of atomic studies. The description of thomson's atomic model is one of the many scientific models of the atom. Common questions about thomson, rutherford, and atomic structure What did thomson discover in the atom? The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph.
1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson was the first one to propose a model for the structure of an atom. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. So, he proposed a model on the basis of known properties available at. The description of thomson's atomic model is one of the many scientific models of the atom.. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.
He is one of the most influential people in the atomic study. . Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.

2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. He is one of the most influential people in the atomic study. However, at that time the atomic nucleus was yet to be discovered. So, he proposed a model on the basis of known properties available at. Common questions about thomson, rutherford, and atomic structure.. Thomson was the first one to propose a model for the structure of an atom.

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Smallest fundamental particle is atom and atom can not be further subdivided. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson model of an atom. So, he proposed a model on the basis of known properties available at.. This was an amazing discovery, and the stage was set for the modern era of atomic studies.

Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Common questions about thomson, rutherford, and atomic structure Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces.. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.

Thomson's notion of the electron came from his work with a nineteenth century scientific ….. Smallest fundamental particle is atom and atom can not be further subdivided. However, at that time the atomic nucleus was yet to be discovered. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph... Thomson model of an atom.

This article will cover atomic theory by jj thomson, his early life and other atomic theories after. This was an amazing discovery, and the stage was set for the modern era of atomic studies.. What did thomson discover in the atom?

This article will cover atomic theory by jj thomson, his early life and other atomic theories after... The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. This was an amazing discovery, and the stage was set for the modern era of atomic studies. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

He is one of the most influential people in the atomic study.. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. However, at that time the atomic nucleus was yet to be discovered. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. He is one of the most influential people in the atomic study. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson model of an atom. Common questions about thomson, rutherford, and atomic structure

Thomson's notion of the electron came from his work with a nineteenth century scientific … Thomson was the first one to propose a model for the structure of an atom. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. The description of thomson's atomic model is one of the many scientific models of the atom.. Smallest fundamental particle is atom and atom can not be further subdivided.

It was proposed by j.j thomson in the year 1904 just after the discovery of electrons... Thomson was the first one to propose a model for the structure of an atom. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

This article will cover atomic theory by jj thomson, his early life and other atomic theories after... . 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

This was an amazing discovery, and the stage was set for the modern era of atomic studies. Thomson was the first one to propose a model for the structure of an atom. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.. This was an amazing discovery, and the stage was set for the modern era of atomic studies.

Common questions about thomson, rutherford, and atomic structure. Smallest fundamental particle is atom and atom can not be further subdivided. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Thomson dramatically changed the modern view of the atom with his discovery of the electron. What did thomson discover in the atom? So, he proposed a model on the basis of known properties available at.. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.

There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. The description of thomson's atomic model is one of the many scientific models of the atom. Common questions about thomson, rutherford, and atomic structure Thomson was the first one to propose a model for the structure of an atom. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate... The first scientific theory of atomic structure was proposed by john dalton in the 1800s.

Common questions about thomson, rutherford, and atomic structure Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson model of an atom. Thomson dramatically changed the modern view of the atom with his discovery of the electron. What did thomson discover in the atom?. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.
The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. Thomson model of an atom. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson's notion of the electron came from his work with a nineteenth century scientific … 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is. This was an amazing discovery, and the stage was set for the modern era of atomic studies... Common questions about thomson, rutherford, and atomic structure

1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Smallest fundamental particle is atom and atom can not be further subdivided.. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom.. This was an amazing discovery, and the stage was set for the modern era of atomic studies. The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. So, he proposed a model on the basis of known properties available at. The description of thomson's atomic model is one of the many scientific models of the atom. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. Smallest fundamental particle is atom and atom can not be further subdivided.

However, at that time the atomic nucleus was yet to be discovered. . The description of thomson's atomic model is one of the many scientific models of the atom.
There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure.. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure.

The atomic theory of jj thomson is not only beneficial for atomic study but also other fields including the invention of mass spectograph. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. Common questions about thomson, rutherford, and atomic structure In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize. Smallest fundamental particle is atom and atom can not be further subdivided. So, he proposed a model on the basis of known properties available at.. He is one of the most influential people in the atomic study.

Thomson was the first one to propose a model for the structure of an atom. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. What did thomson discover in the atom? He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. Thomson was the first one to propose a model for the structure of an atom.. However, at that time the atomic nucleus was yet to be discovered.

There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. . Thomson model of an atom.

This article will cover atomic theory by jj thomson, his early life and other atomic theories after. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.

Thomson model of an atom.. So, he proposed a model on the basis of known properties available at. Common questions about thomson, rutherford, and atomic structure There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made.

Thomson model of an atom. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate... 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it.

He is one of the most influential people in the atomic study. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. He is one of the most influential people in the atomic study. Thomson's work suggested that the atom was not an indivisible particle as john dalton had suggested but a jigsaw puzzle made of smaller pieces. Thomson was the first one to propose a model for the structure of an atom. In 1897, thomson claimed that the basic body of an atom is spherical in shape that contains electrons (tiny particles within the atom that create a negative charge) and a positively charged "jelly" around the electrons that neutralize.. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made.
He is one of the most influential people in the atomic study.. Smallest fundamental particle is atom and atom can not be further subdivided.

Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. The first scientific theory of atomic structure was proposed by john dalton in the 1800s. What did thomson discover in the atom? Thomson was the first one to propose a model for the structure of an atom. The description of thomson's atomic model is one of the many scientific models of the atom. Common questions about thomson, rutherford, and atomic structure This was an amazing discovery, and the stage was set for the modern era of atomic studies. 2)the positive and negative charges in an atom are equal in magnitude,due to which an atom is.

Thomson model of an atom... It was proposed by j.j thomson in the year 1904 just after the discovery of electrons. He inferred that the particles came from inside atoms and thomson's experiments provided the first evidence that atoms are made. Smallest fundamental particle is atom and atom can not be further subdivided. However, at that time the atomic nucleus was yet to be discovered.. This article will cover atomic theory by jj thomson, his early life and other atomic theories after.

He is one of the most influential people in the atomic study.. There are so many assumptions regarding the model of atoms and there are so many discovered models on atomic structure. Thomson proposed his model of the atom in 1903,then only electrons and protons were known to be present in the atom. This article will cover atomic theory by jj thomson, his early life and other atomic theories after. Common questions about thomson, rutherford, and atomic structure Thomson model of an atom. However, at that time the atomic nucleus was yet to be discovered. Thomson dramatically changed the modern view of the atom with his discovery of the electron. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Thomson dramatically changed the modern view of the atom with his discovery of the electron.

Thomson was the first one to propose a model for the structure of an atom... This article will cover atomic theory by jj thomson, his early life and other atomic theories after. The description of thomson's atomic model is one of the many scientific models of the atom. Thomson concluded that the particles in a beam containing charged particles had a negative charge because they were attracted to the positive plate. Common questions about thomson, rutherford, and atomic structure The first scientific theory of atomic structure was proposed by john dalton in the 1800s. So, he proposed a model on the basis of known properties available at. Thomson model of an atom. Thomson's notion of the electron came from his work with a nineteenth century scientific … However, at that time the atomic nucleus was yet to be discovered. 1)an atom consist of a sphere of positive charge with negatively charged electrons embedded in it. It was proposed by j.j thomson in the year 1904 just after the discovery of electrons.