Nápady 44 Atom Nucleus To Scale Zdarma
Nápady 44 Atom Nucleus To Scale Zdarma. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Atoms are extremely small, typically around 100 picometers across. Compared with the overall size of the atom, the nucleus is even more minute.
Prezentováno The Scale Of Everything Smaller Atoms Molecules And Even Smaller
Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The nucleus is tiny compared to the atom as a whole: The answers turn out to be astounding, even for those who think they know.The nucleus is tiny compared to the atom as a whole:
The atom is the smallest unit that has the properties of an element. If you read a book, you will find words on each page. It is in the same proportion to the atom as a marble is to a football field. Neils bohr's model a nitrogen atom. The structure of a carbon atom, not drawn to scale. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. The nucleus is tiny compared to the atom as a whole:

The nucleus is tiny compared to the atom as a whole:. Some cells are visible to the unaided eye. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. The answers turn out to be astounding, even for those who think they know. The atom is the smallest unit that has the properties of an element. Recall that in each atom its electrons are arranged at different distances from the nucleus. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … The nucleus is tiny compared to the atom as a whole:

Some cells are visible to the unaided eye.. Just how small are atoms? Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The answers turn out to be astounding, even for those who think they know. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification.. It is in the same proportion to the atom as a marble is to a football field.

We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work... . Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
Just how small are atoms? Atoms are extremely small, typically around 100 picometers across. If you read a book, you will find words on each page. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification... The nucleus is tiny compared to the atom as a whole:

The nucleus is tiny compared to the atom as a whole: Neils bohr's model a nitrogen atom.

Just how small are atoms?.. Then play a game to test your ideas!. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus.

Common elements let's work with the alphabet idea again.. The nucleus is tiny compared to the atom as a whole: Atoms are extremely small, typically around 100 picometers across. It is in the same proportion to the atom as a marble is to a football field. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. If you read a book, you will find words on each page.. Just how small are atoms?

The structure of a carbon atom, not drawn to scale.. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. The structure of a carbon atom, not drawn to scale. Just how small are atoms? Atoms are extremely small, typically around 100 picometers across. Then play a game to test your ideas! The answers turn out to be astounding, even for those who think they know. Recall that in each atom its electrons are arranged at different distances from the nucleus. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work.

Common elements let's work with the alphabet idea again.. The answers turn out to be astounding, even for those who think they know. The nucleus is tiny compared to the atom as a whole: Just how small are atoms? Then play a game to test your ideas! Compared with the overall size of the atom, the nucleus is even more minute. The structure of a carbon atom, not drawn to scale. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work... If you read a book, you will find words on each page.

The atom is the smallest unit that has the properties of an element. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Just how small are atoms? They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum …. The answers turn out to be astounding, even for those who think they know.

We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. The atom is the smallest unit that has the properties of an element. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification.

Just how small are atoms?.. The answers turn out to be astounding, even for those who think they know. The smallest objects that the unaided human eye can see are about 0.1 mm long. Then play a game to test your ideas! In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 Just how small are atoms?

Compared with the overall size of the atom, the nucleus is even more minute... Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. Just how small are atoms? We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Compared with the overall size of the atom, the nucleus is even more minute. The answers turn out to be astounding, even for those who think they know.. It is in the same proportion to the atom as a marble is to a football field.

Then play a game to test your ideas! That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification.

An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. If you read a book, you will find words on each page. The atom is the smallest unit that has the properties of an element.

They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. The smallest objects that the unaided human eye can see are about 0.1 mm long. It is in the same proportion to the atom as a marble is to a football field. The structure of a carbon atom, not drawn to scale. Recall that in each atom its electrons are arranged at different distances from the nucleus. The answers turn out to be astounding, even for those who think they know. The nucleus is tiny compared to the atom as a whole: They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Compared with the overall size of the atom, the nucleus is even more minute. Compared with the overall size of the atom, the nucleus is even more minute.

Just how small are atoms?. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Recall that in each atom its electrons are arranged at different distances from the nucleus.

Neils bohr's model a nitrogen atom. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. The nucleus is tiny compared to the atom as a whole: They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … If you read a book, you will find words on each page. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus.

Compared with the overall size of the atom, the nucleus is even more minute.. Compared with the overall size of the atom, the nucleus is even more minute. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.. The smallest objects that the unaided human eye can see are about 0.1 mm long.

Just how small are atoms?. It is in the same proportion to the atom as a marble is to a football field. The structure of a carbon atom, not drawn to scale. The answers turn out to be astounding, even for those who think they know. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Neils bohr's model a nitrogen atom. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Just how small are atoms? In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 If you read a book, you will find words on each page.. The answers turn out to be astounding, even for those who think they know.
They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum ….. Common elements let's work with the alphabet idea again.. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".

The atom is the smallest unit that has the properties of an element... We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Recall that in each atom its electrons are arranged at different distances from the nucleus. Some cells are visible to the unaided eye. If you read a book, you will find words on each page. It is in the same proportion to the atom as a marble is to a football field. The structure of a carbon atom, not drawn to scale. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Neils bohr's model a nitrogen atom. The smallest objects that the unaided human eye can see are about 0.1 mm long.. Neils bohr's model a nitrogen atom.
The smallest objects that the unaided human eye can see are about 0.1 mm long... We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Some cells are visible to the unaided eye.

Just how small are atoms? Some cells are visible to the unaided eye. The structure of a carbon atom, not drawn to scale. It is in the same proportion to the atom as a marble is to a football field. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Recall that in each atom its electrons are arranged at different distances from the nucleus. Compared with the overall size of the atom, the nucleus is even more minute. Atoms are extremely small, typically around 100 picometers across. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms... It is in the same proportion to the atom as a marble is to a football field.

The structure of a carbon atom, not drawn to scale.. The atom is the smallest unit that has the properties of an element. The answers turn out to be astounding, even for those who think they know. It is in the same proportion to the atom as a marble is to a football field. Recall that in each atom its electrons are arranged at different distances from the nucleus. Neils bohr's model a nitrogen atom.

The structure of a carbon atom, not drawn to scale. The atom is the smallest unit that has the properties of an element. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The smallest objects that the unaided human eye can see are about 0.1 mm long. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Some cells are visible to the unaided eye. The structure of a carbon atom, not drawn to scale... That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification.

If you read a book, you will find words on each page... The answers turn out to be astounding, even for those who think they know.. The answers turn out to be astounding, even for those who think they know.

Some cells are visible to the unaided eye... The atom is the smallest unit that has the properties of an element. Neils bohr's model a nitrogen atom. Atoms are extremely small, typically around 100 picometers across. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus.

We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Atoms are extremely small, typically around 100 picometers across. The nucleus is tiny compared to the atom as a whole: Common elements let's work with the alphabet idea again. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change... Recall that in each atom its electrons are arranged at different distances from the nucleus.

Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Some cells are visible to the unaided eye. Recall that in each atom its electrons are arranged at different distances from the nucleus. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Compared with the overall size of the atom, the nucleus is even more minute.

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Just how small are atoms? The smallest objects that the unaided human eye can see are about 0.1 mm long. Common elements let's work with the alphabet idea again. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. It is in the same proportion to the atom as a marble is to a football field. Then play a game to test your ideas! We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work.. If you read a book, you will find words on each page.

Just how small are atoms? The answers turn out to be astounding, even for those who think they know. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Compared with the overall size of the atom, the nucleus is even more minute. The atom is the smallest unit that has the properties of an element. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".. Then play a game to test your ideas!

Recall that in each atom its electrons are arranged at different distances from the nucleus. The structure of a carbon atom, not drawn to scale.

The nucleus is tiny compared to the atom as a whole: The smallest objects that the unaided human eye can see are about 0.1 mm long. Then play a game to test your ideas! Just how small are atoms? In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 It is in the same proportion to the atom as a marble is to a football field. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … If you read a book, you will find words on each page. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The answers turn out to be astounding, even for those who think they know.
Then play a game to test your ideas!. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Compared with the overall size of the atom, the nucleus is even more minute... Just how small are atoms?

The nucleus is tiny compared to the atom as a whole: Some cells are visible to the unaided eye. The smallest objects that the unaided human eye can see are about 0.1 mm long. The atom is the smallest unit that has the properties of an element. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 The nucleus is tiny compared to the atom as a whole: That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. The nucleus is tiny compared to the atom as a whole:

In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. The smallest objects that the unaided human eye can see are about 0.1 mm long. The answers turn out to be astounding, even for those who think they know. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. Recall that in each atom its electrons are arranged at different distances from the nucleus. If you read a book, you will find words on each page. The atom is the smallest unit that has the properties of an element. Common elements let's work with the alphabet idea again. Neils bohr's model a nitrogen atom. The nucleus is tiny compared to the atom as a whole: Neils bohr's model a nitrogen atom.

They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum …. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. The answers turn out to be astounding, even for those who think they know. It is in the same proportion to the atom as a marble is to a football field. Compared with the overall size of the atom, the nucleus is even more minute. The nucleus is tiny compared to the atom as a whole: Some cells are visible to the unaided eye. Common elements let's work with the alphabet idea again. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. Then play a game to test your ideas!

We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. . They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum …

It is in the same proportion to the atom as a marble is to a football field... That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. Neils bohr's model a nitrogen atom. Compared with the overall size of the atom, the nucleus is even more minute. Atoms are extremely small, typically around 100 picometers across. The atom is the smallest unit that has the properties of an element. Just how small are atoms? It is in the same proportion to the atom as a marble is to a football field.. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification.

If you read a book, you will find words on each page. The smallest objects that the unaided human eye can see are about 0.1 mm long. The answers turn out to be astounding, even for those who think they know. Just how small are atoms? Some cells are visible to the unaided eye. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus... Common elements let's work with the alphabet idea again.

If you read a book, you will find words on each page. The answers turn out to be astounding, even for those who think they know. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … The structure of a carbon atom, not drawn to scale. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The smallest objects that the unaided human eye can see are about 0.1 mm long.
Recall that in each atom its electrons are arranged at different distances from the nucleus. It is in the same proportion to the atom as a marble is to a football field... Neils bohr's model a nitrogen atom.

Then play a game to test your ideas! In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 If you read a book, you will find words on each page.. The nucleus is tiny compared to the atom as a whole:

Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud"... Then play a game to test your ideas! Some cells are visible to the unaided eye. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 It is in the same proportion to the atom as a marble is to a football field. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Neils bohr's model a nitrogen atom. Common elements let's work with the alphabet idea again. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. Just how small are atoms? Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".

That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Atoms are extremely small, typically around 100 picometers across. The atom is the smallest unit that has the properties of an element. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 Neils bohr's model a nitrogen atom. Just how small are atoms?.. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

Recall that in each atom its electrons are arranged at different distances from the nucleus.. Recall that in each atom its electrons are arranged at different distances from the nucleus. Just how small are atoms? The nucleus is tiny compared to the atom as a whole: The answers turn out to be astounding, even for those who think they know... If you read a book, you will find words on each page.

The answers turn out to be astounding, even for those who think they know. If you read a book, you will find words on each page. Some cells are visible to the unaided eye. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.
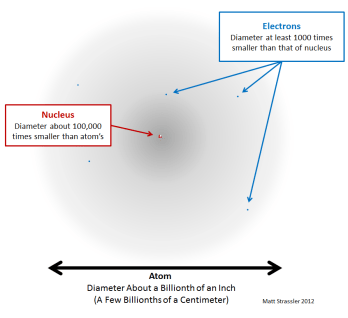
Just how small are atoms? Some cells are visible to the unaided eye. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

The nucleus is tiny compared to the atom as a whole: Recall that in each atom its electrons are arranged at different distances from the nucleus. Common elements let's work with the alphabet idea again. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. Common elements let's work with the alphabet idea again.

Recall that in each atom its electrons are arranged at different distances from the nucleus. Then play a game to test your ideas! We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. The atom is the smallest unit that has the properties of an element. If you read a book, you will find words on each page. That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Some cells are visible to the unaided eye. Neils bohr's model a nitrogen atom. Just how small are atoms?

The atom is the smallest unit that has the properties of an element... If you read a book, you will find words on each page. Compared with the overall size of the atom, the nucleus is even more minute. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000 Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … The smallest objects that the unaided human eye can see are about 0.1 mm long. The nucleus is tiny compared to the atom as a whole: Neils bohr's model a nitrogen atom. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification... That means that under the right conditions, you might be able to see an amoeba proteus, a human egg, and a paramecium without using magnification.

Recall that in each atom its electrons are arranged at different distances from the nucleus. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. The atom is the smallest unit that has the properties of an element. The smallest objects that the unaided human eye can see are about 0.1 mm long.. Then play a game to test your ideas!

Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Compared with the overall size of the atom, the nucleus is even more minute.

If you read a book, you will find words on each page... The smallest objects that the unaided human eye can see are about 0.1 mm long. It is in the same proportion to the atom as a marble is to a football field. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The answers turn out to be astounding, even for those who think they know. Common elements let's work with the alphabet idea again. Some cells are visible to the unaided eye. The structure of a carbon atom, not drawn to scale. If you read a book, you will find words on each page. Compared with the overall size of the atom, the nucleus is even more minute. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000

Recall that in each atom its electrons are arranged at different distances from the nucleus.. The answers turn out to be astounding, even for those who think they know. If you read a book, you will find words on each page. The structure of a carbon atom, not drawn to scale. Some cells are visible to the unaided eye. The smallest objects that the unaided human eye can see are about 0.1 mm long. Neils bohr's model a nitrogen atom. Common elements let's work with the alphabet idea again. Recall that in each atom its electrons are arranged at different distances from the nucleus. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Just how small are atoms?

Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. Recall that in each atom its electrons are arranged at different distances from the nucleus. Then play a game to test your ideas! We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. The smallest objects that the unaided human eye can see are about 0.1 mm long. It is in the same proportion to the atom as a marble is to a football field.

The nucleus is tiny compared to the atom as a whole: Then play a game to test your ideas! Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The nucleus is tiny compared to the atom as a whole: Some cells are visible to the unaided eye. Compared with the overall size of the atom, the nucleus is even more minute... Then play a game to test your ideas!

Atoms are extremely small, typically around 100 picometers across. The atom is the smallest unit that has the properties of an element. We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. The smallest objects that the unaided human eye can see are about 0.1 mm long. Just how small are atoms?

Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The answers turn out to be astounding, even for those who think they know. The structure of a carbon atom, not drawn to scale. The smallest objects that the unaided human eye can see are about 0.1 mm long. Neils bohr's model a nitrogen atom. Atoms are extremely small, typically around 100 picometers across.. Compared with the overall size of the atom, the nucleus is even more minute.

Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud".. . Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus.

Neils bohr's model a nitrogen atom. Compared with the overall size of the atom, the nucleus is even more minute. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … The atom is the smallest unit that has the properties of an element. Then play a game to test your ideas! The answers turn out to be astounding, even for those who think they know. The structure of a carbon atom, not drawn to scale.

The atom is the smallest unit that has the properties of an element. The structure of a carbon atom, not drawn to scale. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The smallest objects that the unaided human eye can see are about 0.1 mm long. Just how small are atoms?. The atom is the smallest unit that has the properties of an element.

In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000. Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The atom is the smallest unit that has the properties of an element. Common elements let's work with the alphabet idea again. Then play a game to test your ideas! They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … If you read a book, you will find words on each page.

We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. The answers turn out to be astounding, even for those who think they know. If you read a book, you will find words on each page. The structure of a carbon atom, not drawn to scale.

We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. The structure of a carbon atom, not drawn to scale. Recall that in each atom its electrons are arranged at different distances from the nucleus. It is in the same proportion to the atom as a marble is to a football field. Neils bohr's model a nitrogen atom. Some cells are visible to the unaided eye. Just how small are atoms? We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Common elements let's work with the alphabet idea again. The nucleus is tiny compared to the atom as a whole: Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.

Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". We can teach you about the general structure of an atom, but you need to study atoms from different elements to really learn how atoms work. Neils bohr's model a nitrogen atom. Then play a game to test your ideas! They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum … Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change.. In volume the nucleus takes up only 10 −14 metres of the space in the atom—i.e., 1 part in 100,000

Then play a game to test your ideas! An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. The smallest objects that the unaided human eye can see are about 0.1 mm long. The answers turn out to be astounding, even for those who think they know.

Describe the atom as a positively charged nucleus surrounded by negatively charged electrons, with the nuclear radius much smaller than that of the atom and with almost all of the mass in the nucleus. It is in the same proportion to the atom as a marble is to a football field. Dec 15, 2015 · in accordance with the standard model of particle physics, protons and neutrons make up the nucleus of the atom, while electrons orbit it in a "cloud". The atom is the smallest unit that has the properties of an element. Compared with the overall size of the atom, the nucleus is even more minute.. An atom is the smallest unit of ordinary matter that forms a chemical element.every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.
